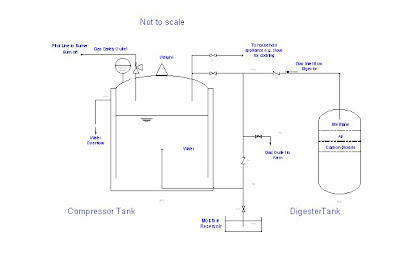
Our objective is to increase the purity of methane in the gas by removing the other contaminant gases, especially CO2 and H2S which is hazardous to the environment and health of people. For effective uses of the biogas, it must be enriched with methane such that the energy value of the gas is increased to give better efficiency.
Industrial method for Absorption of water
One of the most common technique is to employ the absorption with water or otherwise known as ‘water scrubbing’ technique. The principle of this technique exploits the solubility of carbon dioxide, which is better than methane, hence allowing the carbon dioxide to be absorbed better in water. It is noted that the solubility of carbon dioxide increases with pressure so the separation is better conducted at a higher pressure.
The industrial process can be seen in the following simplified scheme:

In this process, the biogas is pressurized before being fed to the bottom of the absorption column where water is fed from the top. A counter-current operation is commonly employed due to better effectiveness. The water containing the absorbed carbon dioxide and a smaller amount of methane is sent to the flash tank where gas is regenerated by de-pressurizing and returned to the absorption column. Regeneration of water is performed by stripping it with air in the desorption column, the stripper. Gas exiting the stripper contains methane losses since methane is slightly soluble in water in the first place.
Absorption with water process
Absorption with water is purely a physical process. This implies that the absorption process does not incorporate chemical reaction. Instead, the mass transfer operation theory is prevalent in this process. The mass transfer from the gas to the liquid phase can be described by the two film theory. This model assumes steady state; hence it provides as a good approximation and to certain good accuracy and allows its mathematical expressions to be relatively easy to comprehend.
• Two-film theory
According to the two-film theory, the resistance to the mass transfer can be described with one or two stagnant films, the gas and liquid film. Since the solubility of the gas follows the Henry’s Law and Henry’s constant of carbon dioxide is large, this implies that the solubility of carbon dioxide is very small and the concentration gradient in the liquid phase is very large. Because of this, the significant resistance for the mass transfer is in the liquid phase and the gas film resistance and gas film itself can be neglected. As such, if the process is controlled by the rate of mean transfer through the liquid film (liquid phase controlled system).
Under these conditions, the total transfer rate of the component A (methane) respective component B (carbon dioxide) from the gas to the liquid phase in the differential volume at the absorption column (scrubber) is described by the two equations below:
Nomenclature
Ci Molar concentration of component i (mol/m3) t top of the column
F Molar rate of gas (mol/s) Ptot Total Pressure (Pa)
QL Liquid rate (m3/s) HA Henry’s constant, (Pa m3/mol)
V Volume of the column (m3)
L Liquid phase
kL0 Mass transfer coefficient (m/s)
a Specific surface area (m2/m3)
Note: Since our design will not be incorporating the flash tank and stripper, their sizing equations will not be further discussed.
We can employ the use of Henry’s law to calculate the equilibrium concentration of gases in a liquid like water. It is noted that the lower the value of the Henry’s constant, the more soluble the gas. The polarity and molecular weight of the gas strongly affects its solubility, with a more polar and higher-molecular-weight gases being more soluble.
Equilibrium
Equilibrium between gas in the solution and in the vapour phase governs the limit to which how much gas can be transferred. The rate at which the gas is transferred into the liquid is governed by kinetics. Equilibrium is affected by properties of gas, temperature, dissolved solids and the partial pressure of gas. For a fixed set of conditions, the equilibrium concentration of a gas in water is proportional to the partial pressure of the substance in the gaseous phase. This relationship is linear at low partial pressures and is represented by the Henry’s law.
Kinetics
We refer to the two-film model proposed by Lewis and Whitman in 1924. Other models that have been used to explain the gas transfer theory include the penetration model by Higbie and the surface renewal model by Danckwertz. However, the two-film model is recommended as it simple and referred to mot frequently.
This research touches on the rate of mass transfer of a volatile substance from water to air which is generally proportional to the difference between the concentrations of the substance in the solution at the system temperature (Henry’s law). The relationship is expressed as follows:
M=KLa (C*-C)
Where:
M= rate of mass transfer (lb/hr/ft3 or kg/hr/m3)
KLa= overall mass transfer coefficient (hr-1)
C*= equilibrium concentration of the gas in the liquid (lb/ft3 or kg/m3)
C= bulk liquid-phase concentration (lb/ft3 or kg/m3)
• Driving force for mass transfer is the difference between the equilibrium and bulk liquid-phase concentrations for the gas.
• The equilibrium conditions are defined by Henry’s law
• The overall mass coefficient kLa is a function of the gas, the process used for gas transfer and the physical parameters, such as temperature and dissolved solids. It is mainly controlled by the liquid-phase resistance.
• Gas transfer processes should be designed to maximize the liquid film mass transfer rate.
Source: Gas purification (Chapter 6- Water as an Absorbent for Gas Impurities) Referex Engineering E-Books.
The advantages of water as an absorbent for gas impurities are its availability and low cost. As such, water can be applicable for large treatment volumes because solvent losses are difficult to avoid in such installations. Water does not require a tight system, and can be used in simple scrubbing units with less concern over leakage and frequently on a once-through basis with the rich solution being discarded. Water may also be applicable to the washing of high-pressure gases where the solubility of an impurity such as CO2 which is only sparingly soluble at low pressure, is brought up to an economically high level by the high CO2 partial pressure. Impurities like CO2 may form acids in aqueous solution; therefore, the prevention of corrosion may become a problem to tackle.
In summary, the advantages of using water over using solvents like monoethanolamine solutions are:
-simple plant design
-no heat load
-Inexpensive solvent
-solvent not reactive with COS, O2 and other possible trace constituents.
Packed Tower Design
This research book does a more on pollution control techniques which require high efficiency and good removal of CO2. It is found that a packed column is commonly used to increase the efficiency of the removal. Parameters such as elevated pressure could help to increase the absorption of CO2 in the process.
It is found that the absorption of carbon dioxide in water has been shown to be almost entirely liquid-film controlled due to the low solubility of CO2. Research on the CO2-H2O system has been conducted to determine the liquid-film resistance to mass transfer when various packings are used. To support this, the work of Cooper et al. (1941) is particularly useful as it employs commercial size packing (2 by 2 by ¼ in. steel raschig rings).
Lines of equal mGM/LM are plotted.
Where:
m is the slope of the equilibrium line, ye/x.
GM is the molar gas velocity, lb moles/ (hr)(sq ft).
LM is the molar liquid velocity, lb moles/ (hr)(sq ft).
This parameter is known as the stripping factor and it represents the ratio of the slope of equilibrium line to the slope of the operating line (normally <1.0 for practical absorption towers and 2.0 for stripping towers). Complete CO2 removal from the gas is effected with excess water.
Data of Sherwood and Holloway for 2-in ceramic rings are included for comparison with that of steel rings. The effective VG (superficial gas velocity) indicated for the ceramic-ring curve represents a value corrected for the difference in free volume between the ceramic and steel rings. The data of Sherwood and Holloway are of general value in estimating HL or kLa for lower liquid-flow rate region. Correlations that apply include:

The above equations are provided as means for calculating the liquid film coefficient, kL and the height of the transfer unit, HL. In CO2 absorption, it is assumed that the gas film resistance is assumed to be of negligible importance.
Note that in a packed tower design, flooding must be considered.
Water scrubbing has been proposed for the removal of CO2 from methane produced by anaerobic digestion. The first pilot unit for this type of process is called the Binax system. Water scrubbing is attractive for this type of application due to its relatively low capital cost in small sizes, simplicity of operation and maintenance, and use of readily available non hazardous absorbent.
Corrosion
Water can become quite acidic when appreciable quantities of carbon dioxide are absorbed. This results in corrosion problems. Due to the operation at near ambient temperature, the low temperature is a favorable factor for corrosion to occur. Oxygen especially accelerates the corrosion of metal by carbon dioxide. Solutions to this problem are to add potassium dichromate to the water, the use of stainless steel in areas of high turbulence, and the application of protective coatings to the interior of the absorber and other vessels. An important thing to note is that water exposed to light may also form algae.
Hydrogen Sulphide removal by absorption in Water
Hydrogen sulphide is appreciably more soluble in water than carbon dioxide. The solubility of hydrogen sulphide in water at moderate pressures has been determined by Wright and Maass (1932). Equilibrium gas and liquid compositions can be calculated from Henry’s law coefficients.
Experimentally determined vapour-liquid equilibrium data for system hydrogen sulphide-carbon dioxide-methane-water at pressures ranging from atmospheric to 1, 014 psia and temperatures from 85oF to 115oF have been reported by Froning et al. (1964). These authors found that the equilibrium constants K can be represented by the following equations:
Update: We discussed the various research with Mr Kon, and have found that we have veered into a complicated route and thus have selected a more simplified yet practical way of sizing the vessel required. Despite this however, we have gained rather valuable knowledge on the various ways of removing CO2 from biogas and their calculations and hence, has resulted in further enhancing our evaluation skills.
 0
comments
Sunday, June 21, 2009
0
comments
Sunday, June 21, 2009